Summary
This literature review serves as an in-depth exploration into the metabolic underpinnings of cancer. The authors present a departure from conventional cancer treatments like chemotherapy and radiation and instead advocate for a multi-faceted approach, comprising of nutritional ketosis, metabolic drugs, and Hyperbaric Oxygen Therapy (HBO2T).
Focusing on the Warburg effect, mitochondrial function, and the role of fermentable fuels like glucose and glutamine, the authors introduce the concept of energy metabolism as a pivotal factor in cancer development and progression.
Emphasizing individual metabolic variations, the text suggests that treatment plans could be personalized to optimize efficacy. The authors present the case for targeting cancer at its metabolic core, proposing this method as less toxic and possibly more effective in the long run. Notably, the text does not shy away from discussing the challenges and uncertainties in implementing this approach. This work adds a valuable perspective to the existing scientific discourse on cancer treatment.
The study was funded by the National Institutes of Health, American Institute of Cancer Research, the Boston College Expense Fund, and the Scivation and Office of Naval Research.
This article explains cancer and how it can be treated in a different way. Normally, doctors use things like chemotherapy and radiation to treat cancer, which can be really hard on the body. This article suggests that scientists should look at how cancer cells get their energy, which happens in a part of the cell called the mitochondria. Some people refer to the mitochondria as the power center of the cell. This article considers how this energy center may hold the answers to how cancer develops so that we can stop this growth. Just like how we need food for energy, cancer cells need certain kinds of energy to grow. The article talks about three main types of energy cells rely on: glucose, a type of sugar; glutamine, an amino acid; and ketones, which are made from broken down fats.
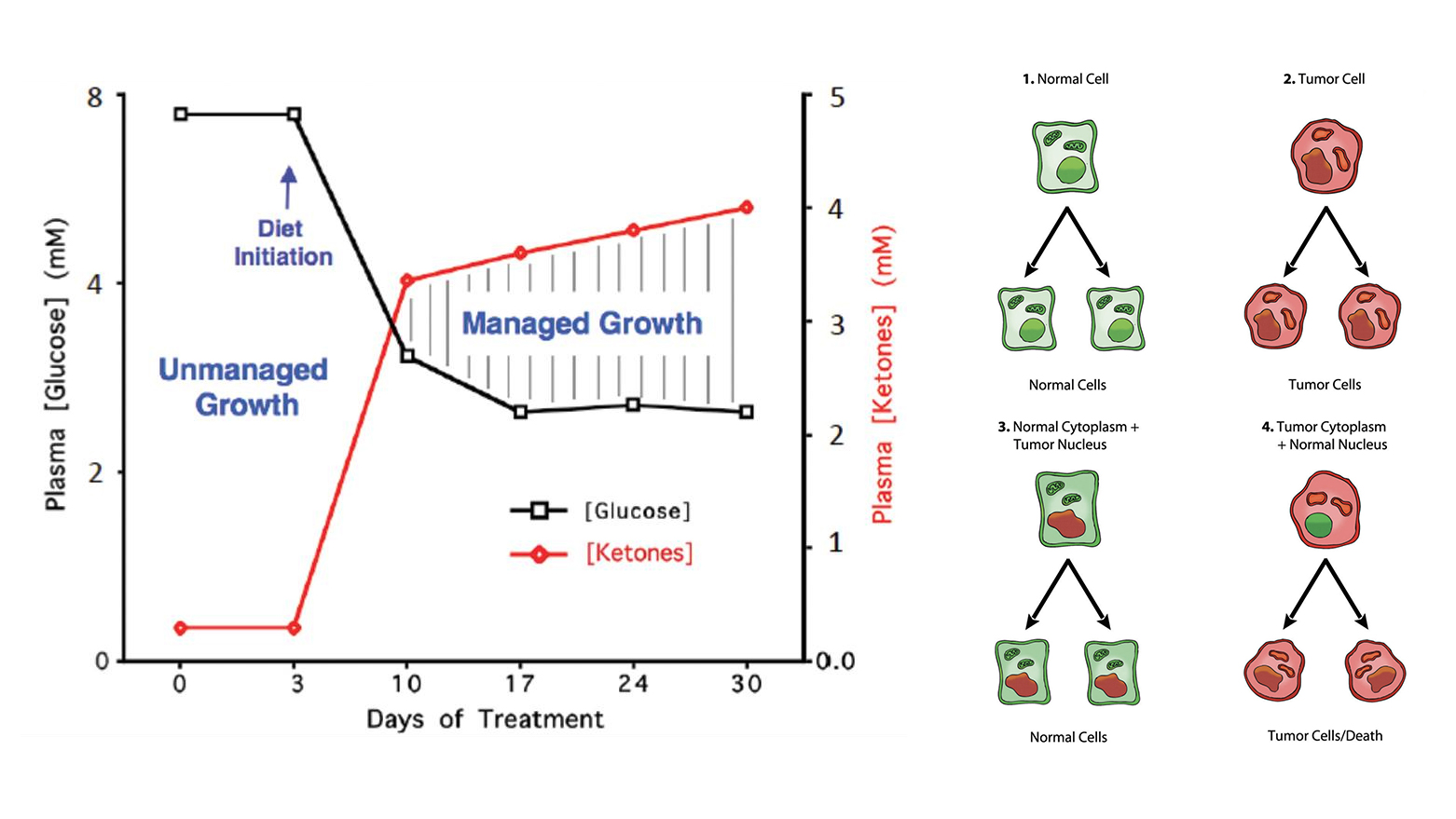
The article suggests that we can change the kinds of energy available to cancer cells by switching the energy sources we offer the cells through our diet. Cancer cells need glucose and glutamine to grow, but they cannot grow on ketones. Human cells can thrive on ketone. By eating a to a Ketogenic Diet, which eliminates sugar and is high in fats we can give our cells the energy they need to be healthy while removing the energy sources that allow the cancer cells to grow. The article also suggests that in addition to this special diet, cancer patients on a ketogenic diet may benefit from using Hyperbaric Oxygen Therapy, where a person breathes in pure oxygen. This treatment helps to destroy cancer cells. However, it works along with the diet, and may not be beneficial if the person is still eating sugar and carbohydrates which produce glucose.
Because cancer cells have different ways of producing energy, the article says that to really beat cancer, you may need to use other medications that specifically target how cancer cells make energy. So, it’s like creating a full-on attack plan against cancer from different angles. This is done in a cycle, using different methods at different times.
The article also explains the importance of making the treatment personal, because everyone is different. The authors suggest that we could fine-tune the treatment based on each person’s body to make it work better. They also say that some advanced cancer treatments, like surgery, could be used as a last resort to clean up any leftover cancer cells. The goal of this metabolic therapy is to stop the growth of the cells and shrink down the number of cancer cells already in the body.
Although more research is needed, this new perspective on treating cancer could be a good path forward.
The study was funded by the National Institutes of Health, American Institute of Cancer Research, the Boston College Expense Fund, and the Scivation and Office of Naval Research.
--------- Original ---------
Emerging evidence indicates that cancer is primarily a metabolic disease involving disturbances in energy production through respiration and fermentation. The genomic instability observed in tumor cells and all other recognized hallmarks of cancer are considered downstream epiphenomena of the initial disturbance of cellular energy metabolism. The disturbances in tumor cell energy metabolism can be linked to abnormalities in the structure and function of the mitochondria. When viewed as a mitochondrial metabolic disease, the evolutionary theory of Lamarck can better explain cancer progression than can the evolutionary theory of Darwin. Cancer growth and progression can be managed following a whole body transition from fermentable metabolites, primarily glucose and glutamine, to respiratory metabolites, primarily ketone bodies. As each individual is a unique metabolic entity, personalization of metabolic therapy as a broad-based cancer treatment strategy will require fine-tuning to match the therapy to an individual’s unique physiology.
The article delves into a different approach to understanding and treating cancer. Rather than focusing solely on traditional treatments like chemotherapy or radiation, it explores how cancer cells get their energy from glucose (a type of sugar) and glutamine (an amino acid). These are described as the "fuels" that help cancer cells grow and spread. The authors suggest that cutting off these fuel sources could be a key way to stop cancer's progression.
One way to do this is through a Ketogenic Diet (KD), which is high in fats and low in carbohydrates, thereby reducing glucose levels. When combined with Hyperbaric Oxygen Therapy (HBO2T), which involves breathing in pure oxygen in a controlled environment, this approach aims to create an unfriendly environment for cancer cells. Essentially, the diet and the oxygen therapy serve to "starve" the cancer cells of their energy sources, making it difficult for them to grow and survive.
However, the article acknowledges that some cancer cells can adapt and find other ways to sustain themselves. To counter this, the authors propose the use of specific metabolic drugs that can target and disrupt the alternative ways cancer cells might get their energy. They refer to this multi-pronged strategy as a "Press-Pulse" approach, attacking the cancer cells from different angles to ensure their elimination.
Each individual is unique in their metabolism, so tailoring the treatment to fit the patient's specific physiological needs could make the therapy more effective. Advanced molecular therapies could serve as a "clean-up" strategy for any remaining cancer cells after the primary metabolic treatment.
The article also touches on the role of human cytomegalovirus in cancer progression and the metabolic shifts in the body during the treatment.
The authors advocate for the broader application of this metabolic approach, arguing that it could not only be effective in treating cancer but also beneficial for the patient’s overall health.
In summary, the authors make a strong case for a shift in cancer treatment, focusing more on metabolic therapies that target the energy sources of cancer cells. They suggest that this approach could be less harmful to patients than current methods, and that it should be considered as a viable option for future cancer treatment strategies.
The study was funded by the National Institutes of Health, American Institute of Cancer Research, the Boston College Expense Fund, and the Scivation and Office of Naval Research.
--------- Original ---------
Emerging evidence indicates that cancer is primarily a metabolic disease involving disturbances in energy production through respiration and fermentation. The genomic instability observed in tumor cells and all other recognized hallmarks of cancer are considered downstream epiphenomena of the initial disturbance of cellular energy metabolism. The disturbances in tumor cell energy metabolism can be linked to abnormalities in the structure and function of the mitochondria. When viewed as a mitochondrial metabolic disease, the evolutionary theory of Lamarck can better explain cancer progression than can the evolutionary theory of Darwin. Cancer growth and progression can be managed following a whole body transition from fermentable metabolites, primarily glucose and glutamine, to respiratory metabolites, primarily ketone bodies. As each individual is a unique metabolic entity, personalization of metabolic therapy as a broad-based cancer treatment strategy will require fine-tuning to match the therapy to an individual’s unique physiology.
Emerging evidence indicates that cancer is primarily a metabolic disease involving disturbances in energy production through respiration and fermentation. The genomic instability observed in tumor cells and all other recognized hallmarks of cancer are considered downstream epiphenomena of the initial disturbance of cellular energy metabolism. The disturbances in tumor cell energy metabolism can be linked to abnormalities in the structure and function of the mitochondria. When viewed as a mitochondrial metabolic disease, the evolutionary theory of Lamarck can better explain cancer progression than can the evolutionary theory of Darwin. Cancer growth and progression can be managed following a whole body transition from fermentable metabolites, primarily glucose and glutamine, to respiratory metabolites, primarily ketone bodies. As each individual is a unique metabolic entity, personalization of metabolic therapy as a broad-based cancer treatment strategy will require fine-tuning to match the therapy to an individual’s unique physiology.
Let's start with the truth!
Support the Broken Science Initiative.
Subscribe today →
recent posts
Expanding Horizons: Physical and Mental Rehabilitation for Juveniles in Ohio
Maintaining quality of life and preventing pain as we age.