The ROS hypothesis of obesity
Synopsis
Background
Hypothesis
- A reassessment of insulin sensitivity and insulin resistance
- Polyunsaturated fats limit ROS formation and promote weight gain
- Saturated fats generate appropriate ROS and limit weight gain
- Ketogenic diets: an alternative solution to excessive insulin sensitivity
- Pathological insulin sensitivity in the context of a ketogenic diet
- Linoleic acid in the diet vs linoleic acid in adipocytes
Synopsis
Hypothesis:
Insulin’s metabolic control is mediated through ROS generation, limiting insulin signaling at normal physiologic levels. Failure to limit insulin signaling at physiological levels results in fat storage. Excessive insulin sensitivity — or “pathological insulin sensitivity” — in adipocytes in the presence of insulin signaling increases storage, and this is regulated in part by the number of double bonds in the fatty acids they’re oxidizing. Oxidation of fatty acids produces less ROS with each additional double bond. Failure to generate insulin resistance — or the facilitation of excessive insulin sensitivity — is the underlying cause of obesity.
Why we get fat:
An increase in the consumption of polyunsaturated fats, particularly linoleic acid, promotes excessive insulin sensitivity, resulting in excessive nutrient ingress into cells and consequent adipocyte hypertrophy.
What to do about it:
Decrease consumption of fatty acids that fail to limit insulin signaling at physiological levels (i.e., linoleic acid), and replace them with fatty acids that limit signaling at physiological levels (i.e., saturated fats). An alternative (or complimentary) solution is a very low-carbohydrate or well-formulated ketogenic diet. Since linoleic acid causes weight gain by failing to limit insulin signaling, linoleic acid content becomes less relevant during such diets because there’s less insulin and insulin signaling present. Therefore, there’s effectively no signal to limit.
Background
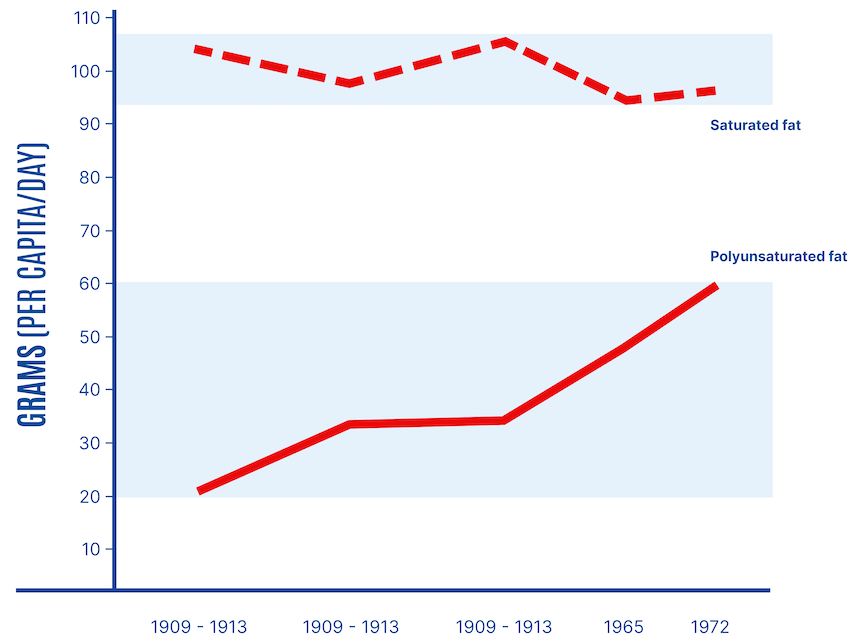
For nearly a century, replacing saturated fats in the diet with polyunsaturated fats has been a cornerstone of dietary advice for Americans and abroad. Following findings by Ancel Keys and others in the late 1950s and early 1960s, the American Heart Association recommended reducing saturated fats and cholesterol intake. These recommendations were first issued in 1961 and institutionalized, replacing saturated fats with polyunsaturated fats to manage cholesterol levels and reduce the risk of coronary heart disease. This advice coincided with increased consumption of seed oils, also called vegetable oils. “Considerable amounts of saturated fat are present in whole milk, cream, butter, cheese, and meat,” the AHA committee explained. “In contrast to the above food fats, many natural vegetable oils, such as corn, cotton and soya, as well as the fat of fish, are relatively low in saturated fats and high in fats of the poly-unsaturated type.” This coincided with a substantial increase in the consumption of polyunsaturated fats (Figure 1).
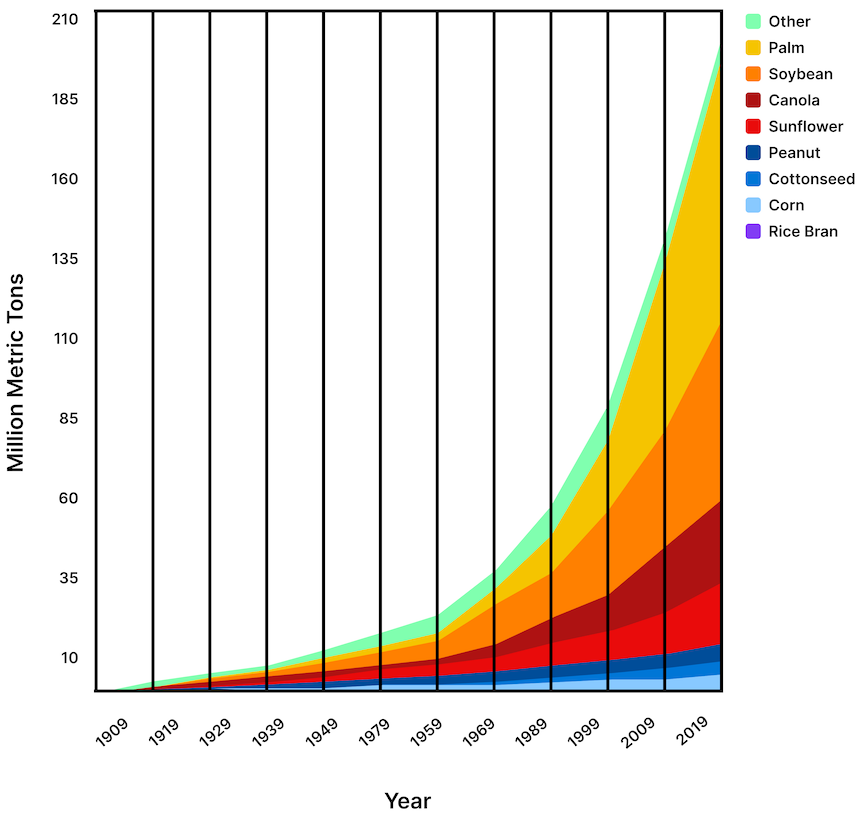
By 2010, Americans were consuming more than three times the amount of polyunsaturated fats, mainly from seed oils containing mostly linoleic acid, compared with a century earlier. Global vegetable oil production has increased more than 16-fold since 1909 and has doubled in the last 20 years. Consumption of soybean oil alone (55% linoleic acid) has grown more than 1,000-fold since 1909 (Figure 2).
Obesity rates over this time have increased similarly.
Further reading and resources:
- Zero Acre, 2022 | Seed Oils as a Driver of Heart Disease
- Nobbs, 2020 | What’s Driving Chronic Disease?
- Blasbalg et al., 2011 | Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century
Hypothesis
A reassessment of insulin sensitivity and insulin resistance
The ROS hypothesis, proposed by Petro Dobromylskyj in 2012,1 The hypothesis was proposed by Petro Dobromylskyj in 2012. He has honed the hypothesis ever since, sharing theses developments in a series of posts on his blog. The “Protons” series comprises 77 posts at the time of this writing (October 28 2024). Perspectives related to the hypothesis are not limited to this series. Many more posts on his blog besides the Protons series contain information and insight relevant to the ROS hypothesis. suggests that consumption of polyunsaturated fats, such as linoleic acid, exacerbates fat storage, whereas saturated fats help limit it. The relatively recent shift in fat types, driven by the fear of saturated fats and the push towards polyunsaturated fats to purportedly reduce heart disease risk, may have inadvertently fueled the obesity epidemic.
The conventional wisdom is that increased insulin sensitivity is a good thing. It allows low excursions of plasma insulin to maintain normal blood glucose levels. On the other hand, insulin resistance is widely accepted as a bad thing. In particular, insulin resistance with compensatory hyperinsulinemia is considered pathological. The more insulin-sensitive and the less insulin-resistant you are, the better.
What exactly do we mean by insulin sensitivity? In what cells and tissues? Is insulin resistance a good thing under certain circumstances? Is insulin sensitivity a bad thing under certain circumstances? One of the ROS hypothesis’s central concepts is the term coined by Petro Dobromylskyj, pathological insulin sensitivity.
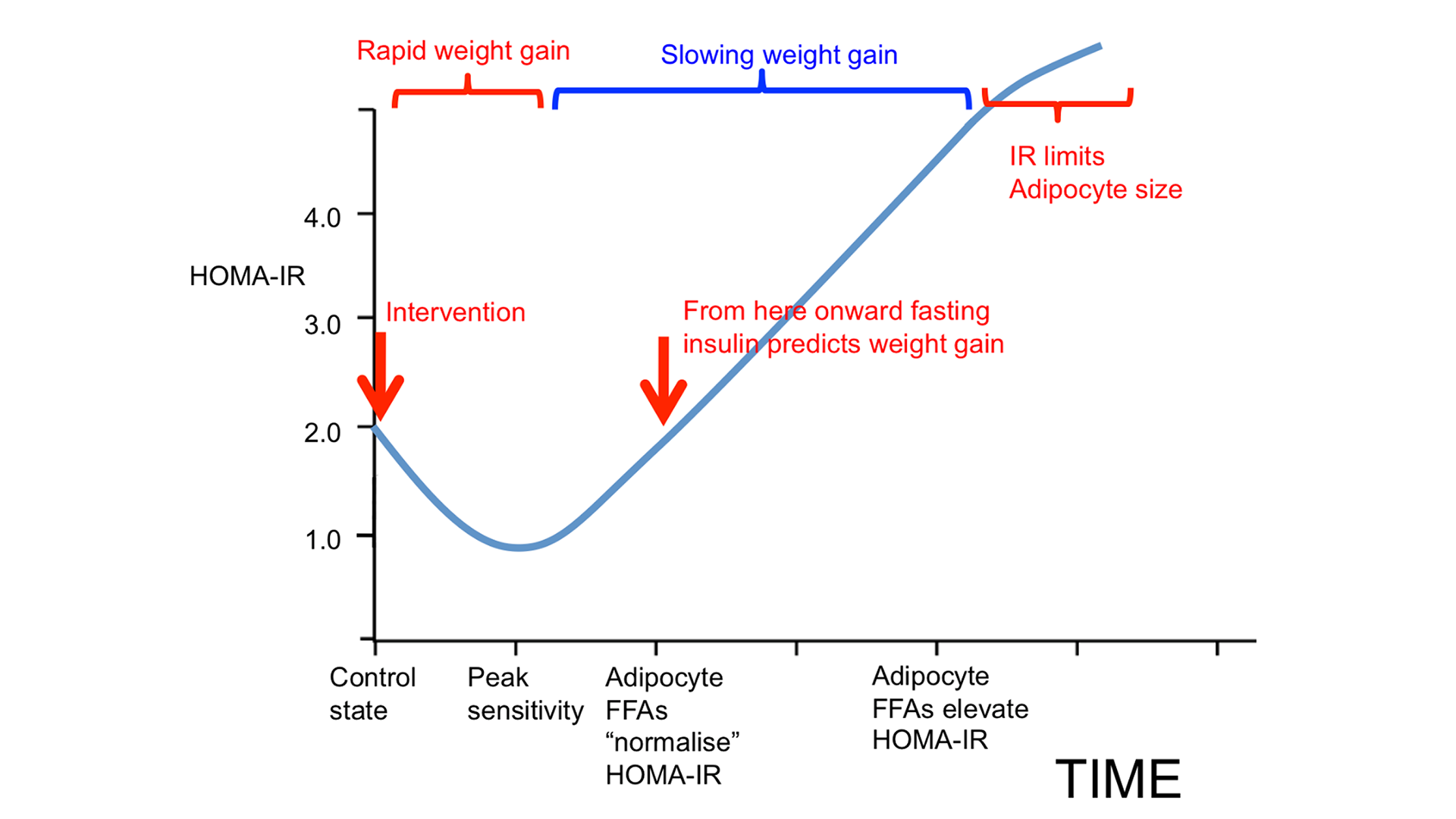
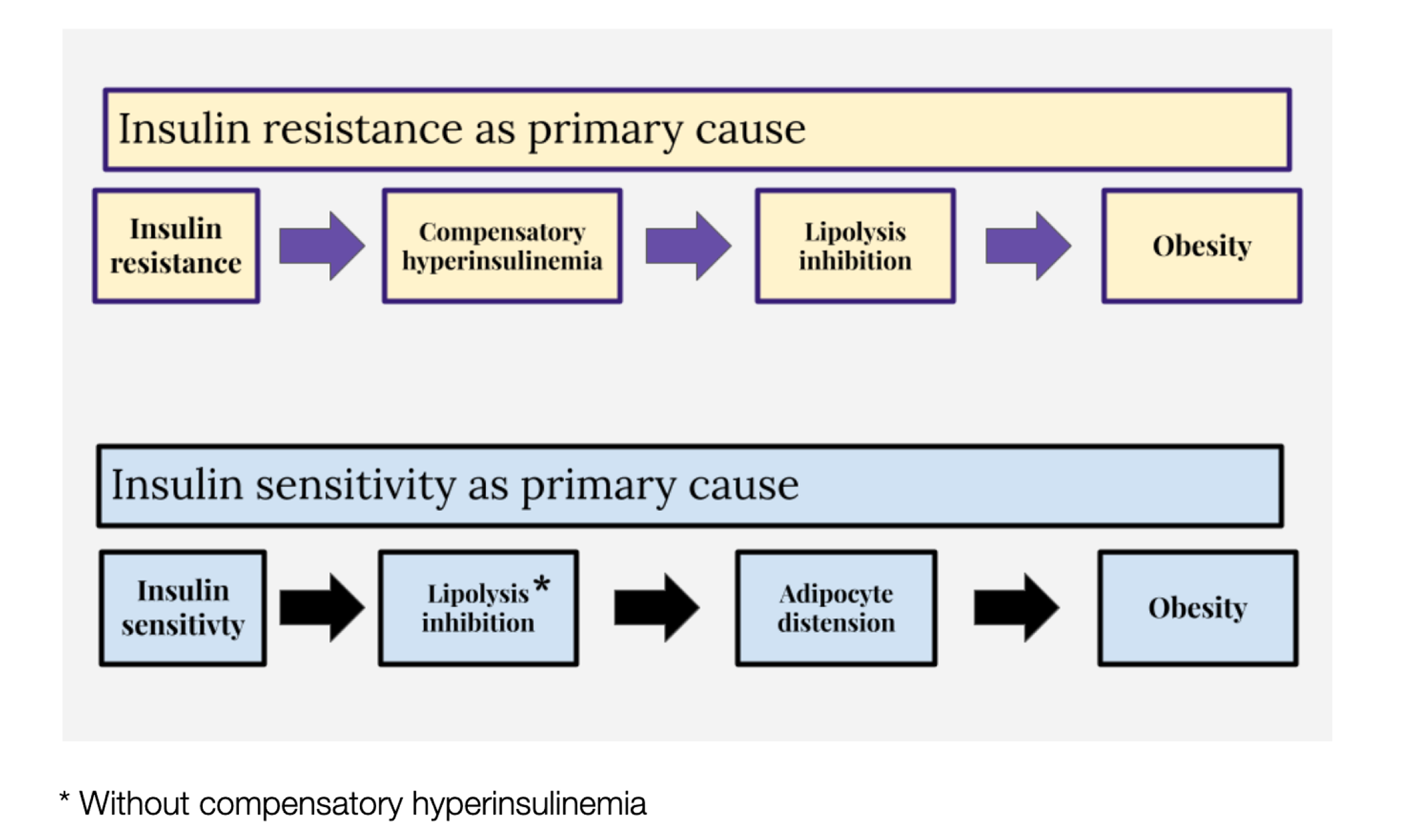
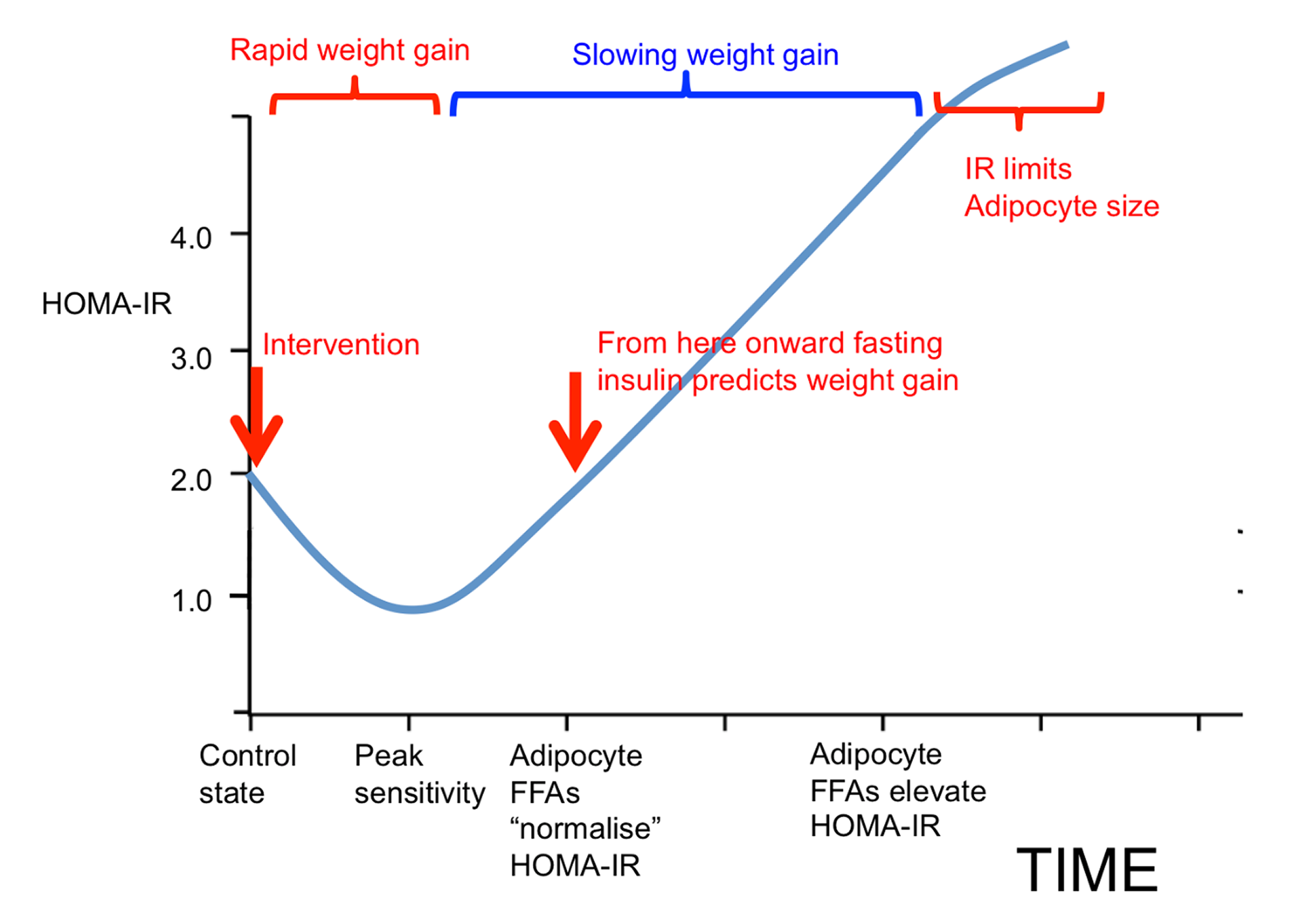
One model of obesity holds that insulin resistance is the underlying cause. If insulin resistance occurs, compensatory hyperinsulinemia follows, especially with the consumption of rapidly digestible carbohydrates. The amount of insulin required to maintain normal blood glucose is in considerable excess of that needed to inhibit lipolysis — the process of breaking down stored fat to mobilize it for energy use. This leads to obesity (Figure 3, upper panel).
From the ROS hypothesis perspective, insulin’s metabolic control is mediated through ROS generation. Failure to limit insulin signaling at normal physiological levels results in fat storage. Failure to generate insulin resistance — or the facilitation of excessive insulin sensitivity — is the underlying cause of obesity (Figure 3, lower panel).
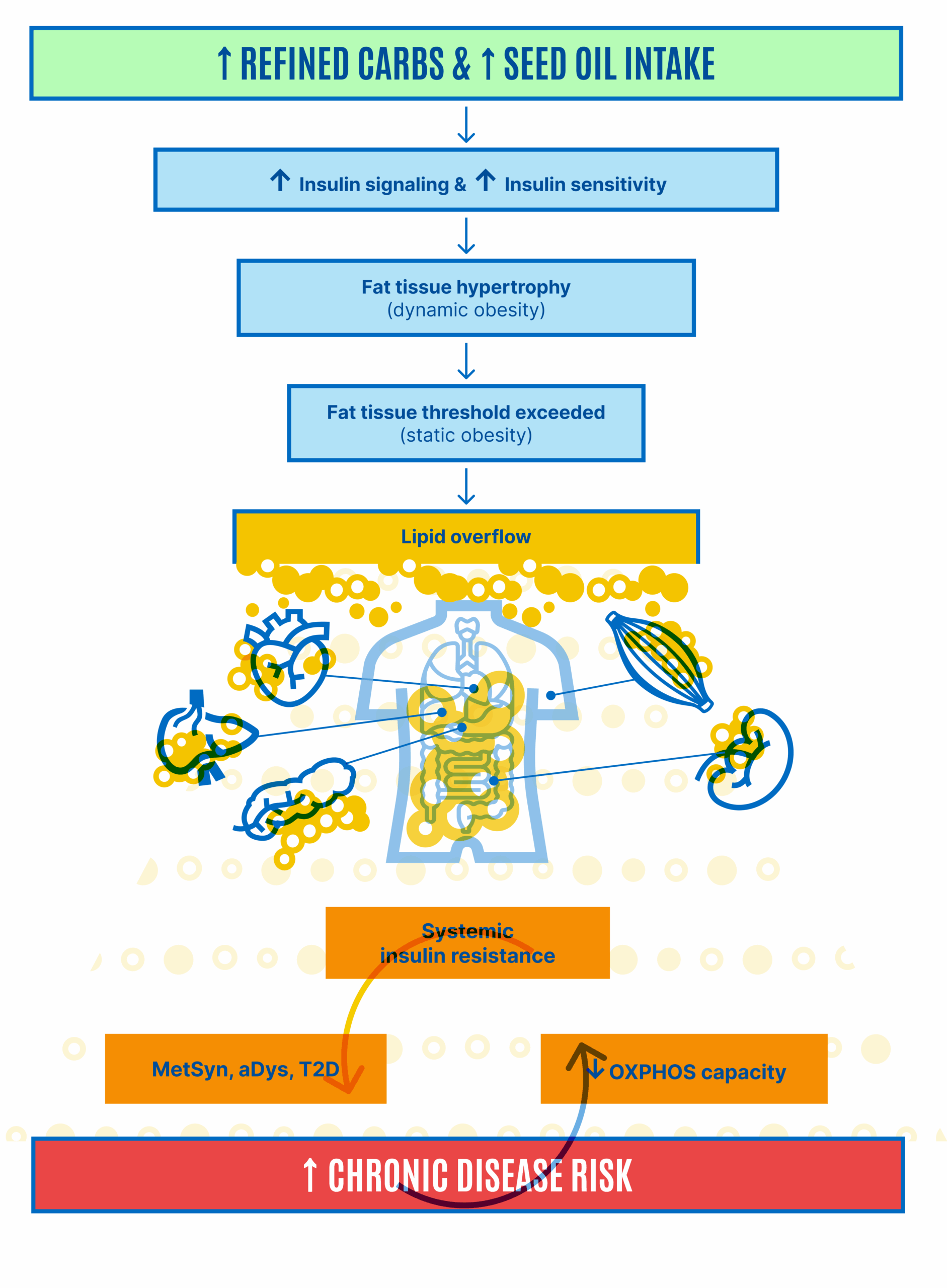
This allows for the inhibition of lipolysis without compensatory hyperinsulinemia. Hyperinsulinemia is not required to inhibit lipolysis if the adipocytes are excessively sensitive to insulin. The result is adipocyte distension. Our fat cells get fatter. Therefore, we get fatter, and this manifests as obesity. This view holds that obesity is a result of a failure to limit insulin signaling to physiologically appropriate levels. As obesity progresses, eventually, there are problems with the loss of free fatty acids from distended adipocytes through increased basal lipolysis, which leads to whole-body or systemic insulin resistance. However, this is a secondary complication of obesity, not the primary driver, according to the ROS hypothesis (Figure 4).
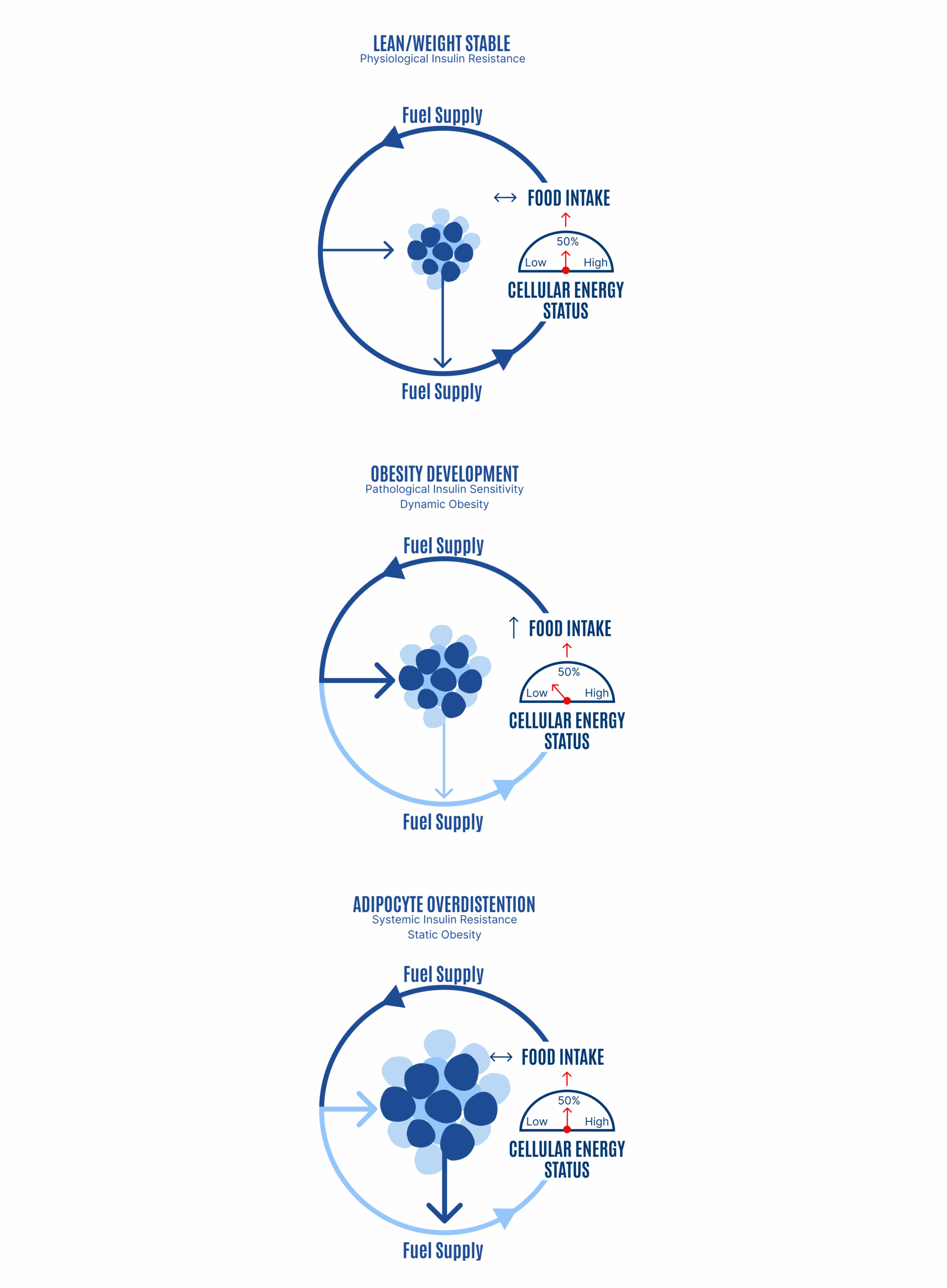
Inadequate ROS production will allow overdistension of adipocytes, which is another way of saying excess loss of metabolic fuels into storage and obesity development. Excessive consumption of polyunsaturated fats, such as linoleic acid, biases the partitioning of metabolic fuels toward storage and a greater proportion of fuel is trapped in the fat tissue. The compensatory response is increased hunger or slowed metabolic rate, or both. This is the dynamic phase of obesity (Figure 5, middle panel). As obesity progresses, adipocytes distend (i.e., adipocyte hypertrophy). As adipocytes distend, they increase their basal lipolysis, which cannot be suppressed by insulin.
Adipocytes distend until the rate of storage into the adipocytes is matched by the increasing rate of basal lipolysis and the FFAs they spill out: a settling point where the competition of forces between mobilization and deposition appears to be in a state of balance (Figure 5, lower panel). The inability of insulin to suppress excessive basal lipolysis also results in systemic insulin resistance. The individual effectively progresses from a period of internal starvation (Figure 5, middle panel) to nutrient overload and lipotoxicity (Figure 5, bottom panel and Figure 6).
Further reading and resources:
- Dobromylskyj, 230628 | Insulin sensitivity makes you fat. Insulin resistance makes you fat. Discuss. (01)
- Dobromylskyj, 230929 | Insulin sensitivity makes you fat. Insulin resistance makes you fat. Discuss. (04) M scores
- MetFix | A primer on the ROS hypothesis of obesity
- MetFix | Webinar with Peter Dobromylskyj
- MetFix | Annotated presentation: ROS signaling controls nutrient ingress into cells | Peter Dobromylskyj
- Eades, 2018 (YouTube) | Dr. Michael Eades – ‘A New Hypothesis of Obesity’
Polyunsaturated fats limit ROS formation and promote weight gain
Viewed in the context of the ROS hypothesis, increasing the consumption of PUFAs results in decreased generation of ROS, which are core signaling molecules. Oxidation of fatty acids produces less ROS with each additional double bond.
A crucial component of the ROS hypothesis is that the FADH2 to NADH ratio (FN ratio) mediates reverse electron transport (RET) through Complex I. RET occurs when electrons are driven backward from the CoQ pool toward Complex I. Here, an electron can be passed to molecular oxygen, generating ROS. This is a highly controlled and fully physiological phenomenon. The ROS ends up as hydrogen peroxide, which, if enough of it is produced, inhibits insulin signaling and nutrient ingress into the cell, a form of localized insulin resistance or cellular satiety. A portion of the brain may detect the nutrient levels present in excess of those being disposed of by peripheral insulin into peripheral cells. As large numbers of peripheral cells become “full” in the manner just described, the brain should pick up the rising level of excess nutrients as the signal to call a halt to hunger mediated by ROS. Based on studies in the brain (also see Further reading and resources), the same pattern emerges: low levels of ROS indicate low energy availability and stimulate hunger; and high levels of ROS indicate high energy availability and stimulate satiety.
Since the mitochondria integrate the total energy availability in a cell, a failure to generate appropriate levels of ROS can result in a failure to develop resistance to insulin-facilitated nutrient ingress into cells. If we don’t limit nutrient ingress into cells — particularly into our fat cells — our fat cells get fatter. This manifests as what we would call obesity at the whole-body level.
The ROS hypothesis posits that ROS generation determines the ability to limit nutrient ingress into cells. If we accept that every double bond in a PUFA blunts this effect, replacing dietary saturated fats with polyunsaturated fats reduces ROS generation. Failure to limit nutrient ingress is a recipe for excess fat accumulation (i.e., obesity).
Further reading and resources:
- Speijer, 2016 | Being right on Q: shaping eukaryotic evolution
- Muller, 2008 | High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates
- Dobromylskyj, 191111 | Protons (51): From peripheral cells to the brain
- Drougard et al., 2015 | Impact of hypothalamic reactive oxygen species in the regulation of energy metabolism and food intake
- Horvath et al., 2009 | Fuel utilization by hypothalamic neurons: roles for ROS
- Benani et al., 2007 | Role for Mitochondrial Reactive Oxygen Species in Brain Lipid Sensing
Saturated fats generate appropriate ROS and limit weight gain
ROS has a physiological role in limiting nutrient ingress, and saturated fats do this better than polyunsaturated fats. The ROS hypothesis suggests that saturated fats in the diet generate ROS at relatively high levels, serving as a signal to stop excessive nutrient ingress at the cellular level, particularly the adipocytes, where most lipids are stored. Inadequate ROS production will allow overdistension of adipocytes, which is another way of saying excess fat accumulation, which is another way of saying obesity.
Since the limitation of adipocyte size depends on ROS generation, the ROS hypothesis suggests that replacing polyunsaturated fats with saturated fats in the diet limits fat accumulation and reduces the risk of obesity.
Other considerations
Ketogenic diets: an alternative solution to excessive insulin sensitivity
The ROS hypothesis of obesity posits that pathological insulin sensitivity is the primary driver of obesity. This is caused by excessive nutrient ingress and storage into adipocytes due to a failure to generate appropriate ROS to limit insulin signaling. The hypothesis emphasizes limiting linoleic acid — an 18-carbon fatty acid with two double bonds — because it is ubiquitous in the modern diet and is likely the most common cause of excessive insulin sensitivity.2 Linoleic acid is also in somewhat of a mitochondrial sweet spot. For example, the omega-3 fatty acid DHA has 22 carbons and six double bonds. DHA should be more obesogenic than linoleic acid, but it’s not. Why? Very long-chain fatty acids like DHA are initially oxidized in peroxisomes, not mitochondria. Omega-3s are also known to exert uncoupling effects on mitochondria, which can ultimately reduce body fat stores and prevent fat accumulation.
If we consider that polyunsaturated fats like linoleic acid cause weight gain by failing to limit insulin’s signaling, what happens on a well-formulated ketogenic diet where circulating insulin levels are low? In other words, if linoleic acid fails to appropriately limit insulin signaling, what if there’s little to no signal to limit?
Studies demonstrate that hypoinsulinemic diets like the ketogenic diet, irrespective of the level of unsaturation of the fatty acids in the diet, allow fat loss or at least do not promote weight gain. There are rodent studies, for example, fed a ketogenic diet called F3666 that provides approximately 15% or more of its kcal as linoleic acid, and these mice do not gain weight, and their weights remain lower than control mice fed standard chow. The same should apply to humans: relatively high polyunsaturated fat ketogenic diets can achieve fat loss, provided the diet keeps insulin levels low.
The ROS hypothesis suggests that the hypoinsulinemic effect of a diet can override or sidestep the high concentration of linoleic acid in the diet. In the absence of insulin, the FN ratio becomes less relevant. The lower the amount of insulin in circulation, the lower the insulin signaling. In other words, if insulin is kept at basal levels, it doesn’t matter how much insulin signaling is inappropriately handled if there’s no signal to handle. What would happen to those mice if more carbohydrates were added to their diet, or humans on a high-PUFA ketogenic diet? The ROS hypothesis predicts the commencement of weight gain because of the insulin signaling that the carbohydrates induce, and the pathological sensitivity to insulin signaling that the linoleic acid elicits.
Further reading and resources:
- MetFix | A primer on the ROS hypothesis of obesity
- Kennedy et al., 2007 | A high-fat, ketogenic diet induces a unique metabolic state in mice
Pathological insulin sensitivity in the context of a ketogenic diet
One caveat to a high-PUFA ketogenic diet is that if the diet is high enough in linoleic acid, the ROS hypothesis of obesity suggests this can lead to inadequate ROS generation and excessive sensitivity to insulin signaling. Generally, glucose, insulin, and insulin signaling are lower on a ketogenic diet. However, there is still some insulin signaling, especially if the diet is not deeply ketogenic and relatively moderate in carbohydrate intake, as far as low-carbohydrate or ketogenic diets are concerned.
In a small crossover trial of 14 participants, one of the phases included a ketogenic diet with 12% of calories from linoleic acid and 10% from carbohydrates. One of the participants had to withdraw because they developed hypoglycemia. This likely didn’t develop due to inadequate glucose production, as the liver can provide adequate glucose through gluconeogenesis. The ROS hypothesis suggests that excessive insulin sensitivity from the linoleic acid in the diet, in the face of insulin signaling triggered by carbohydrate consumption, could produce excessive loss of glucose into cells that don’t require glucose. An underlying concept of a ketogenic diet, or fasting, is that the body should be insulin resistant so that it doesn’t use glucose and is spared for the brain. Physiological insulin resistance under fasting or ketogenic eating is essential to spare glucose for the brain. This mechanism should avoid hypoglycemia or symptomatic hypoglycemia. From the ROS hypothesis point of view, the linoleic acid content of the diet and excessive insulin sensitivity should be considered when implementing or embarking on a diet, including a ketogenic diet.
Further reading and resources:
- Hall et al., 2021 | Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake
- Dobromylskyj, 210131 | Hall and CICO part 3
Linoleic acid in the diet vs linoleic acid in adipocytes
With a little effort, linoleic acid depletion from the diet can be immediate. For most Americans, a substantial reduction in the amount of linoleic acid in the diet can be achieved by avoiding pre-packaged, fat-based products like salad dressing and mayonnaise, and switching cooking oils to those lower in polyunsaturated fats.
However, diets high in linoleic acid also cause it to accumulate in tissues, particularly adipocytes. This is compatible with the ROS hypothesis: excessive nutrient ingress and storage into adipocytes due to a failure to generate appropriate ROS by polyunsaturated fats like linoleic acid. A review of studies measuring the linoleic acid concentration in fat tissue in the US reported nearly a threefold increase from 1959 to 2008, with levels increasing from about 9% to 22%.
Another consideration here is that following a meal. After a meal, chylomicron-TGs from a fat-containing meal are typically the dominant source of fatty acids entering adipocytes and other tissues following a meal, both circulating free fatty acids and VLDL-TGs — which both originated in large part by the mobilization of fatty acids from the fat tissue — can contribute to the nutrient mix that the cells, and mitochondria, see after a meal. So even when there’s little to no exogenous linoleic acid in a meal, there remains the possibility that some quantity of endogenous linoleic acid in circulation can contribute to the FN ratio and, therefore, the level of ROS generation, at the mitochondrial level postprandially. The amount or proportion of linoleic acid contributing likely reflects in part the linoleic acid concentration in adipocytes, so it may be more challenging to generate appropriate ROS for individuals with a high concentration of linoleic acid in their fat tissue.
Linoleic acid depletion from adipocytes requires a longer-term effort than eliminating it from the diet. The proportion of linoleic acid in the diet correlates with the proportion incorporated into fat tissue. The half-life of linoleic acid in adipose tissue is approximately two years.
Further reading and resources:
- Guyenet and Carlson, 2015 | Increase in Adipose Tissue Linoleic Acid of US Adults in the Last Half Century
Dietary suggestions based on the ROS hypothesis
Minimize consumption of fatty acids that fail to limit insulin signaling at physiological levels:
- Reduce consumption of linoleic acid. Aim for about 0.1 g/kg of body weight (bw) per day from linoleic acid (over about 0.3 g/kg-bw/d is considered obesogenic)
- Increase consumption of saturated fats
Alternatively, or in addition, minimize insulin signaling:
- Consume a very-low-carbohydrate (<0.5 g/kg-bw/d) or ketogenic diet
Bob Kaplan is an independent research analyst. Bob previously served as director of research at Early Medical, contributing to The Drive podcast with Peter Attia and its other properties. Kaplan was a researcher at the Nutrition Science Initiative (NuSI), and an exercise physiologist at the University of Nevada, Las Vegas. His current and previous research interests include meta-research, chronic diseases, bioenergetics, exercise physiology, and nutrition.
Support the Broken Science Initiative.
Subscribe today →
2 Comments
Leave A Comment
You must be logged in to post a comment.
recent posts
And more evidence that victory isn’t defined by survival or quality of life
The brain is built on fat—so why are we afraid to eat it?


Can we say saturated fats promote satiety better than polyunsaturated fats?
That’s the argument.