April 2023 article by Undark.
Recent reforms could require companies to perform follow-up studies on drugs that received accelerated approval.
“She [Ramachandran] was surprised to find that, as she recalls, hers was the only independent voice and the only person focused on patients rather than hastening the approval process. ‘The FDA has been increasingly approving new medications more quickly and based on less robust evidence,’ she told a House subcommittee. ‘This has left our patients, and us as prescribers, with greater uncertainty around whether these approved products are truly clinically beneficial and safe.’”
Let's start with the truth!
Support the Broken Science Initiative.
Subscribe today →
recent posts
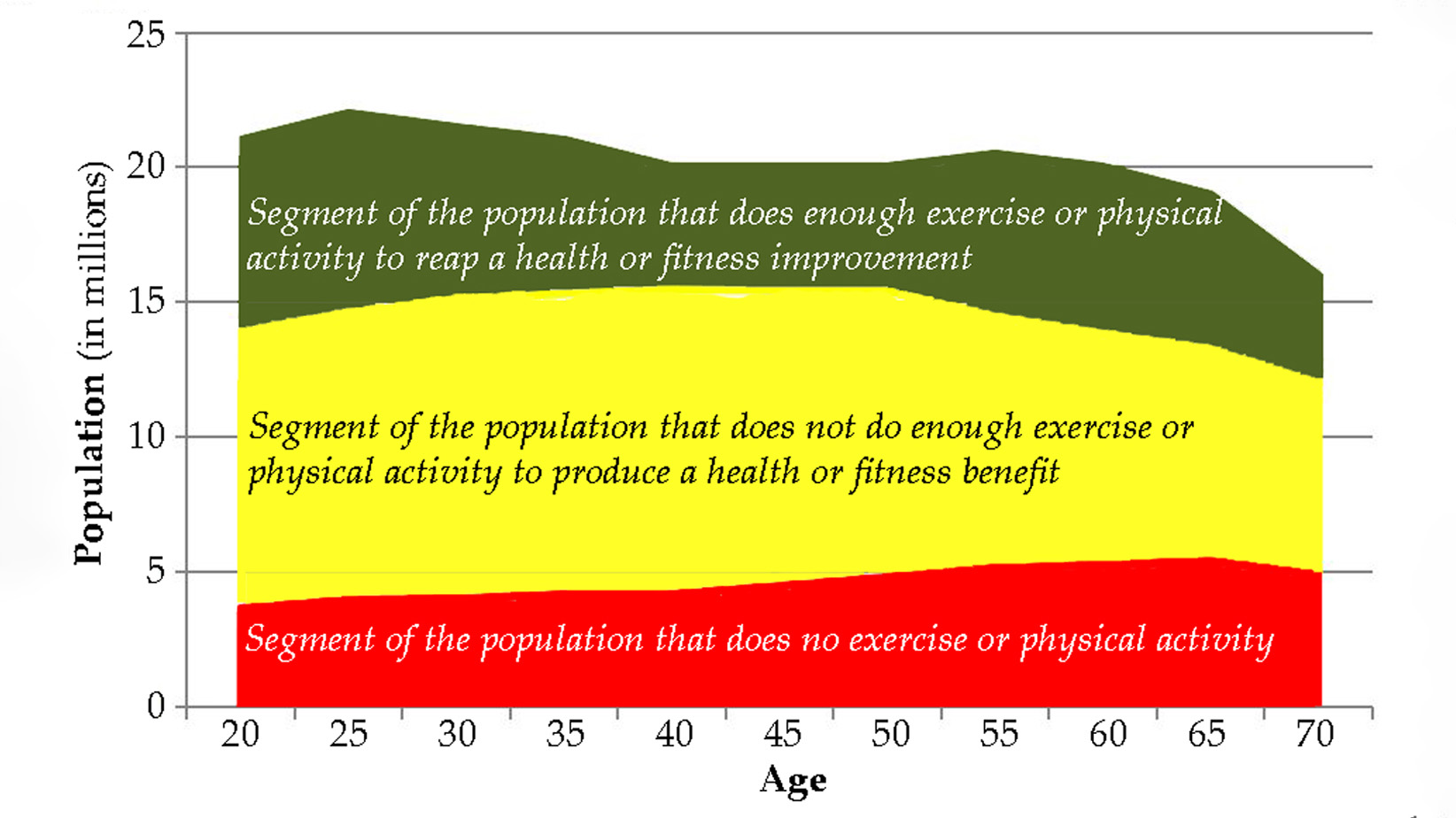
Expanding Horizons: Physical and Mental Rehabilitation for Juveniles in Ohio
Maintaining quality of life and preventing pain as we age.