In the last article I highlighted the growing problem of a replication crisis within science. Increasingly, when people attempt to replicate experiments or trials that have been done by others, they cannot. Not always, but in the majority of cases.
This is a major problem, which is getting worse, but within the realm of placebo-controlled medical trials the replication barrier is multiplied. This is because, once a placebo-controlled trial has demonstrated significant benefit, it can never be repeated. As it would be considered unethical to withhold any treatment that has just been ‘proven’ to be effective.
Would you call this a Catch 22, or double indemnity? Or the perfect crime? Falsify your research once, and it can never be disproved. Or to be more charitable, mess-up your results, and no-one can ever prove otherwise. Your results will stand forever.
Is there anything you do if you suspect that a placebo-controlled trial contains significant errors? Given the impossibility of repeating the study, the only thing left is to search for obvious errors, or anomalies. Always bearing in mind that nothing can be proved beyond any doubt, unless you can find some way of doing the study again.
Bearing this in mind I am going to go back in time to look at the 4S (Scandinavian Simvastatin Survival Study) in more detail. There are a couple reasons for choosing this particular trial.
First, because the 4S study is the most influential, and pivotal statin trial ever done. It pretty much laid the foundations for statins becoming the most prescribed class of drugs, ever. Secondly, it showed by far the greatest benefit from statins in any placebo-controlled study.
It was published over thirty years ago and has never been seriously questioned. Despite the fact that it contains at least one very strange anomaly. For which I have never been able to find an explanation. And the more I look, the stranger the anomaly becomes. In order to fully explain why it is an anomaly, I need to establish some context.
The immediate effect of statins – in all other trials
There have been many, many, studies done on statins. Hundreds. They are not all directly comparable, not by any manner of means. Some were done using placebos, several looked at one statin vs. another. Or, on a high dose vs. low dose of the same statin. A few were carried out in high-risk patients, others done to look at statins in patients with diabetes.
All of which makes comparison between studies somewhat tricky, as you are looking at different populations, and different trial designs. However, there is one consistent finding in all the statin trials (where the data have been made available. Which is that any benefit from taking statins emerges almost immediately.
Below is the cumulative survival curve from the study “mortality across all age groups of individuals with significant coronary disease, including very elderly patients” Published in the Journal of the American college of cardiology.
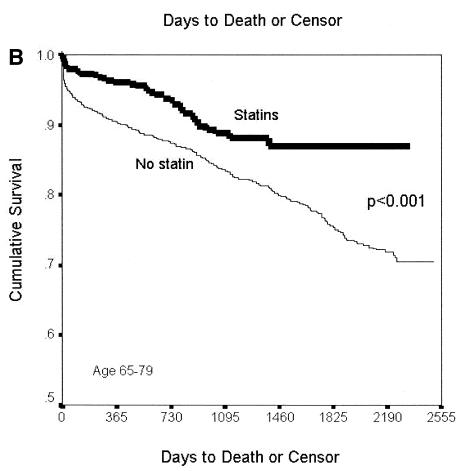
Forget, for now, about the survival rates themselves. Instead, I would like you to focus on the fact that the survival lines separate very early on. Those not taking statins started dying at a higher rate from day one, virtually. (The curves then start coming together again before, for a period of three years, not a single person taking statins dies…. An issue for another time perhaps, where we can discuss the massive bias inherent in this, and other such studies.)
Whilst this study is looking at roughly the same type of population as 4S, it was not a randomized placebo-controlled study. Instead, it looked at the impact of ‘statins vs. no-statins’ on different age-groups, who suffered from significant cardiovascular disease. The graph covers those aged sixty-five to seventy-nine. In short, it is similar to 4S, if not exactly the same.
Below is another ‘survival’ graph again looking at statins vs.no statins. The scale used here is months, not days or years. This graph is also presented ‘upside down’ with mortality gradually rising, rather than survival falling. No one ever presents data that same way, or on the same group of people. (This group was very high risk, they were dialysis patients who had suffered a heart attack, which is why around 50% were dead after four years.)
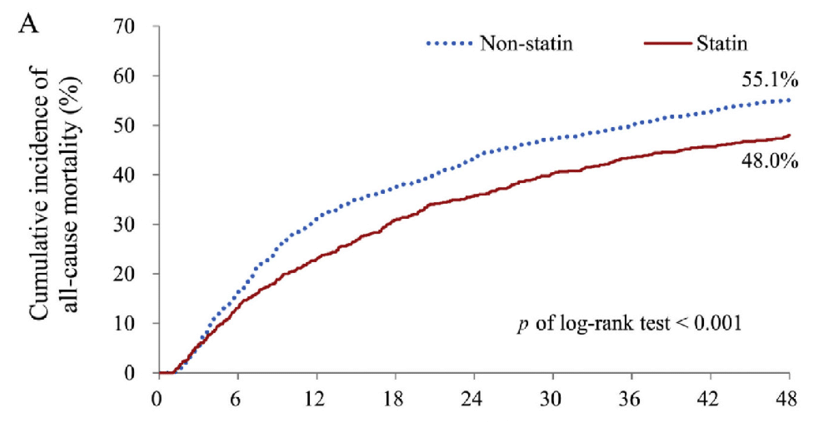
As before, there is a rapid separation in overall mortality, with a clear divergence appearing at around three months. By twelve months the mortality lines are as far apart as they are ever going to get.
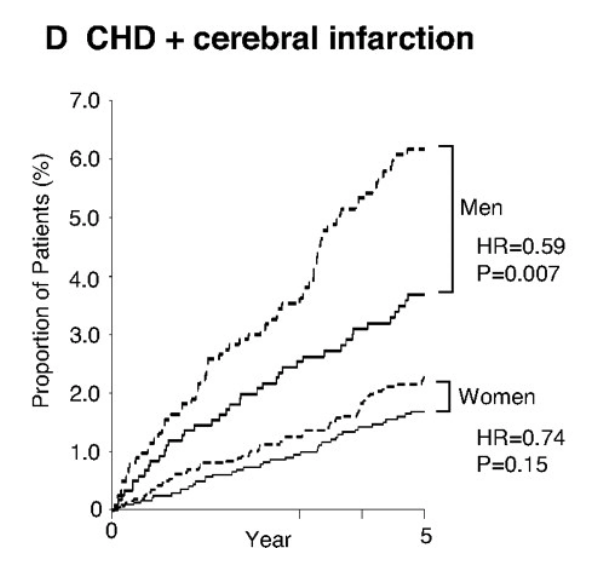
Below is another study looking at the cumulative incidence of coronary heart disease (heart attacks) and strokes in both men and women taking statins, or no-statins. Once again, separation starts immediately. The dotted line(s) represent those taking the placebo.
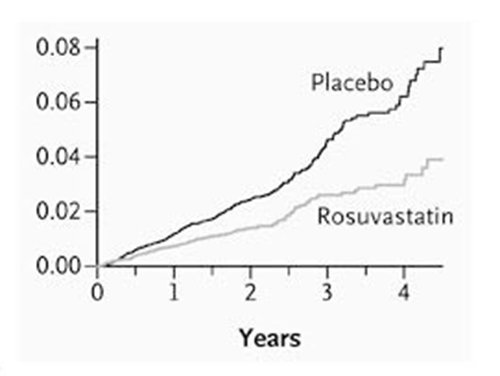
And now for two more graphs, the first from the highly influential JUPITER study. In this study the mortality rate was low, as it was a primary prevention study (no previously known cardiovascular disease i.e. no past strokes or heart attacks.) The x axis covers four years. (The y axis rises in hundredths of a per-cent)
The next graph looks at mortality following the insertion of a stent (per-cutaneous intervention PCI.) As you can see the benefit of statin appears after days, reaching statistical significance after less than a month.
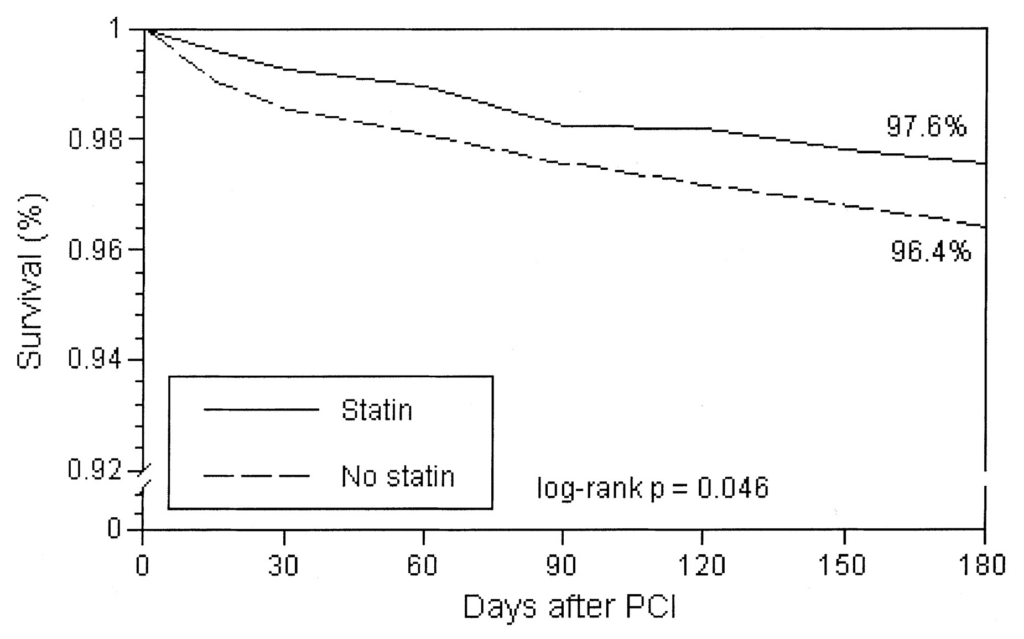
These studies, and these graphs, are looking at different populations, at very different risks of cardiovascular and/or overall death. However, one thing that does stand out is that the benefit from taking statins appears almost immediately. Often within days.
I should make it clear that these five studies are not outliers. The same effect appears in almost every single statin study – when such survival graphs have been published.
Why is this important?
Some more context – How statins are supposed to work.
The reason why statins exist, as a class of drugs, is because they were found to reduce low density lipoprotein levels (LDL levels). This action is often, inaccurately, called ‘cholesterol’ lowering. The two terms are often used interchangeably, just to make life more complicated.
Low density lipoproteins are believed to move out of the bloodstream and into the artery wall, where they clump together causing thickenings and narrowings called atherosclerotic plaques – also known as atherosclerosis. This ‘disease’ has many other, interchangeable names.
The thinking behind the statins trials is that the higher the LDL level the more rapidly the deposition will occur. Therefore, if you lower LDL, then deposition slows, so the progression of atherosclerosis (thickening and narrowing of arteries) will also slow – perhaps stop.
Given this proposed mechanism of action, one thing you would certainly not expect to see would be an immediate benefit on heart attacks and strokes, as atherosclerosis is known to take decades to build up. And only after it has developed will it then lead to heart attacks and strokes. To quote the American Journal of Medicine:
“Atherosclerosis develops over the course of 50 years, beginning in the early teenage years.”
And from the 4S paper itself:
“…Progression of coronary atherosclerotic lesions clearly predicts subsequent coronary events.”
If you slow or stop LDL deposition in the statin arm of a clinical trial it will clearly take some time before this will result in any measurable difference in ‘coronary events.’ How long?… years possibly.
However, as we have just seen, statins demonstrate an immediate benefit. What does this mean? It means that statins must have other, much faster acting effects. Or, to put this another way, statins do not simply lower LDL levels, they are doing many other things at the same time. Some, or all, of which are potentially beneficial. (The technical term for this type of action, with any drug, is ‘pleiotropic’ effects. Actions that a drug has that are in addition to its main mechanism of action.)
Just to start by looking at a potential protective action. Which is that statins have potent anti-coagulant agents (they slow, or prevent, blood clotting.) Here, from the paper “Statins effects on blood clotting, a review.”
“Experimental studies indicate that statins alter blood clotting at various levels. Statins produce anticoagulant effects via downregulation of tissue factor expression and enhanced endothelial thrombomodulin expression resulting in reduced thrombin generation. Statins impair fibrinogen cleavage and reduce thrombin generation.”
There are many other beneficial effects of statins, which could also provide rapid benefit.
Statins are:
- Anti-inflammatory
- Anit-oxidant
- Protective against vascular smooth muscle proliferation (a key part of the ASCVD process)
- Vasodilatory/widen blood vessels – reducing blood pressure
If you were to type in the words statins and pleiotropic effects into Google you get, or I got, 339,000 results. Mostly from peer-reviewed medical journals.
Any, or all of these actions, could lead to immediate benefits on cardiovascular risk. Especially their anticoagulant action. Aspirin, for example, was initially developed to lower temperature and reduce pain. It was then found to have significant anticoagulant actions. On this basis it is given to patients during a heart attack to reduce blood clots growing and blocking arteries.
Aspirin is now well known to act very rapidly in reducing mortality from CV disease. And it works almost immediately. Newer anti-coagulants such as clopidogrel have replaced aspirin in an acute event, along with stents. Of interest here, aspirin survival graphs look almost exactly the same as statins survival curves.
Here is a graph of aspirin (ASA) vs. no aspirin no (ASA) following discharge from hospital following an admission with unstable angina. (More modern anticoagulants cannot be tested against a placebo, for reasons outlined several times in this article.)
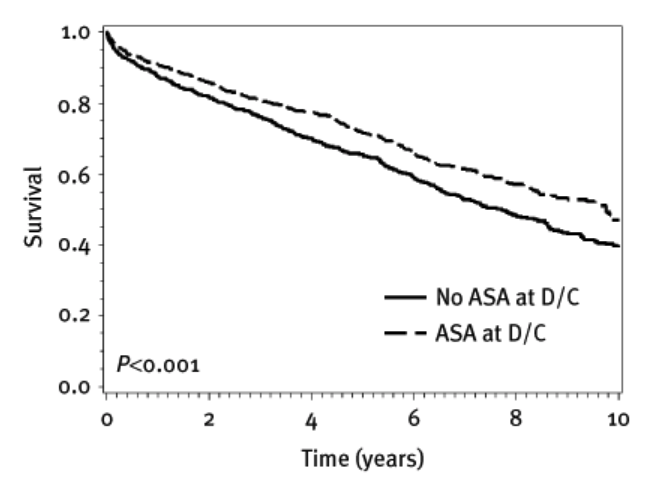
Pulling a few strands together. We know the following:
- Statins demonstrate immediate benefits on CV events.
- This cannot be due to LDL lowering.
- Statins have many other ‘pleiotropic’ actions that could well explain their rapid beneficial effect.
Given all the known pleiotropic effects the finding of immediate benefit with statins is not a surprise. Indeed, it would be a surprise not to see an immediate benefit.
What did the 4S trial show?
Below is the graph from the trial itself. Unlike the other studies, two survival lines are locked together for around eighteen months (I added in the line at ~18 months to make things clearer) then suddenly separate. The placebo line drops, whilst the statin line does not move – for around six months. Which gives us the initial separation.
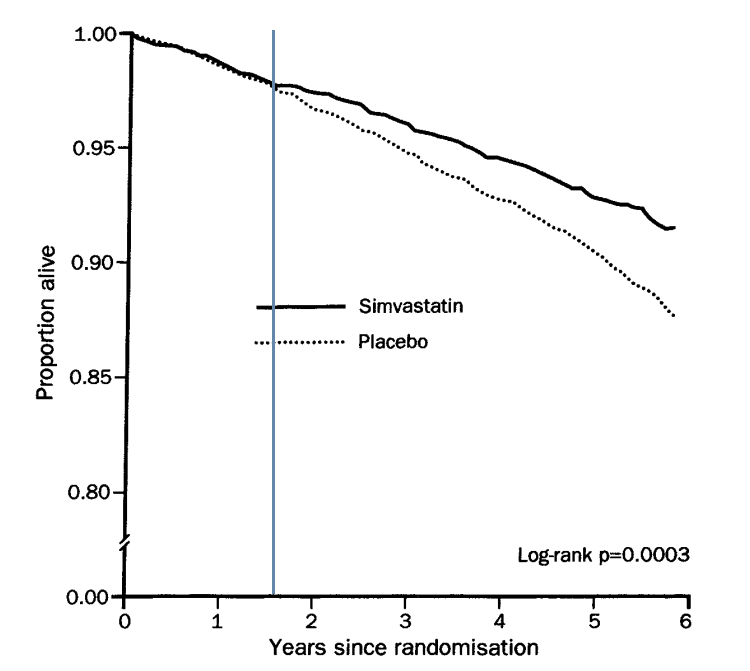
To be as charitable as I can manage, the 4S researchers found something that no-one else found. Namely, that there was no benefit to be seen from taking statins for over eighteen months. Then, quite suddenly, the survival lines separate.
This is an anomaly, and it represents a major flashing red light. (Imagine if they had stopped the trail after eighteen months.)
Changing tack slightly, who did the monitoring, the data processing and the data analysis for the 4S study – using simvastatin, which is a Merck drug.
Monitoring offices, Merck Sharp & Dohme. G Renstrom Moen, J Hylerstedt; V Larsen; S Lillsjo; R Nyberg; C Eriksen, D Fogh Nielsen, T Musliner, R Greguski
Data processing center: Irving K Hwang. Merck Research laboratories, Woodbridge, New Jersey.
Data analysis. T Cook (Merck Research Laboratories).
What else do we know about the many statin trials? Here from Scientific American:
“In 2007, researchers looked at every published trial that set out to explore the benefit of a statin. These are cholesterol lowering drugs which reduce your risk of having a heart attack, they are prescribed in very large quantities…
“This study found 192 trials in total, either comparing one statin against another, or comparing a statin against a different kind of treatment. Once the researchers controlled for other factors (we’ll delve into what this means later), they found that industry-funded trials were twenty times more likely to give results favoring the test drug.”
As I said at the start of this article, there is a replication crisis within science; and there is a replication barrier within placebo controlled clinical trials. In that you can never do these trials again, even if you wanted to. Added to this, there is a further issue with industry funded trials, in that they are remarkably effective at finding benefit from their own drugs, and showing what they are expected to show.
With 4S we have seen the greatest benefit in any of the placebo-controlled statin trial. There was also this very strange anomaly. Before the trial started, the thinking was that it would take some time before any benefit would be seen from taking a statin. And in 4S it did. However, this effect was seen nowhere else. Something that should have raised loud loud alarm bells, not just about the anomaly, but the entire trial itself.
If I were looking for a trial to replicate, I would be very interested in doing 4S again. However, even if I were to find the hundreds of millions of dollars required to do so, I would be stuck. The rules on placebos prohibit any attempt to repeat this trial. Which leaves us where?
REFERENCES:
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S); The Lancet; November 19, 1994.
- Statin therapy is associated with reduced mortality across all age groups of individuals with significant coronary disease, including very elderly patients; Journal of the American College of Cardiology; November 20, 2002.
- Moderate to high intensity statin in dialysis patients after acute myocardial infarction—A national cohort study in Asia; Atherosclerosis; September 27, 2017.
- Usefulness of Pravastatin in Primary Prevention of Cardiovascular Events in Women: Analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA Study); Circulation; January 29, 2008.
- Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein; The New England Journal of Medicine; November 20, 2008.
- Early and Sustained Survival Benefit Associated With Statin Therapy at the Time of Percutaneous Coronary Intervention; Circulation; December 31, 2001.
- The Pathology of Atherosclerosis: Plaque Development and Plaque Responses to Medical Treatment; The American Journal of Medicine; January, 2009.
- Advances in the molecular mechanisms of statins in regulating endothelial nitric oxide bioavailability: Interlocking biology between eNOS activity and L-arginine metabolism; Biomedicine and Pharmacotherapy; February, 2024.
- Trial sans Error: How Pharma-Funded Research Cherry-Picks Positive Results [Excerpt]; Scientific American; February 13, 2013.
Scottish doctor, author, speaker, sceptic
Support the Broken Science Initiative.
Subscribe today →
recent posts
Medical Society Webinar with David Wiss