By Bob Kaplan
Here’s a recent example of some of the important work BSI is helping to fund and make happen:
“Could Cytoplasmic Lipid Droplets be Linked to Inefficient Oxidative Phosphorylation in Cancer?” by Thomas N. Seyfried, Nathan L. Ta, Tomas Duraj, Derek C. Lee, Michael A. Kiebish, Christos Chinopoulos & Gabriel Arismendi-Morillo
As a background to the review, Thomas Seyfried and his colleagues indicate that triglyceride-rich cytoplasmic lipid droplets (LDs) are present in many malignant cancers. There is a debate in the field as to whether the LDs serve as storage depots of fuel via oxidative phosphorylation (OxPhos) or are a consequence of impaired or inefficient OxPhos.
As a background to the background, Seyfried argues — as Otto Warburg did a century before him — for the mitochondrial metabolic theory (MMT) of cancer: that cancer is a mitochondrial metabolic disease, arising from a gradual disruption of energy (ATP) synthesis through OxPhos leading to compensatory ATP synthesis through substrate level phosphorylation. It was Warburg, in the early twentieth century, who observed that, unlike normal (i.e., differentiated) cells, cancer cells ferment glucose (i.e., produce lactate) even in the presence of high levels of oxygen, a characteristic now known as the Warburg effect. However, the vast majority of researchers in the field ascribe to the somatic mutation theory (SMT), that cancer arises from inherited or random somatic mutations in key genes. Also according to the SMT, tumor cells have a growth advantage and are more fit than normal cells.
In their new paper, Seyfried and colleagues review the literature and identify the presence of LDs in most malignant cancers. They have also shown evidence for abnormalities in mitochondrial number, structure, or function in those same cancers, as shown in the accompanying Table. (Most of that evidence can be found in a 2020 review by Seyfried and colleagues.) “Based on the foundational principle of evolutionary biology that structure determines function,” they write, “abnormalities in mitochondrial structure reduce the efficiency of OxPhos.”
The upshot? The well-documented accumulation of LDs and mitochondrial abnormalities seen in a broad range of malignant cancers are not due to an advantageous adaptation of cancer cells for energy use, they are more likely a pathologic consequence of impaired or inefficient OxPhos, according to the authors. “Based on the information reviewed,” they write, “we favor the most parsimonious explanation that the lipid droplets seen in common cancers are linked to insufficient OxPhos.
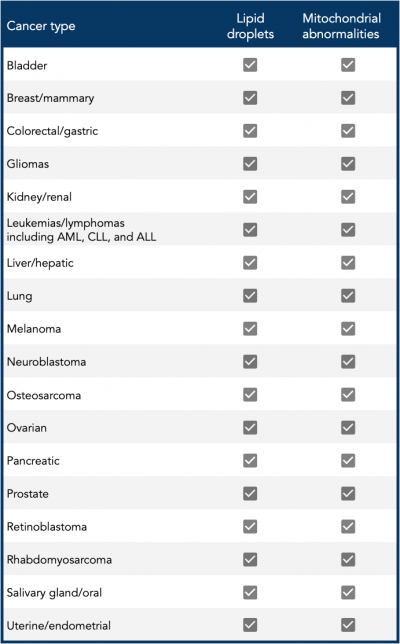
Table | Presence of cytoplasmic lipid droplets and mitochondrial abnormalities in common cancers [credit: Seyfried et al., 2024; Seyfried et al., 2020]
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia
There’s an interesting parallel here between the LD-in-cancer controversy and a phenomenon referred to as the athlete’s paradox. “Endurance athletes have also been reported to contain higher levels of skeletal muscle LDs compared with untrained individuals,” write Filip Kolodziej and Ken O’Halloran in a 2021 review. “Paradoxically, LD content also strongly correlates with a degree of insulin resistance in type 2 diabetics…”
In the case of endurance athletes, LD accumulation is almost assuredly an adaptive response, allowing for a local depot and turnover of highly efficient fuel in cells containing a relatively higher number and higher functioning mitochondria, according to most researchers. (There’s also a distinction of the LD location in athletes, but that’s beyond the scope of the argument here.) A local storage depot of triglycerides for cells that are repeatedly exposed to higher ATP demands. In the case of type 2 diabetes (T2Ds), it is generally accepted that LDs accumulate as a pathologic consequence of impaired or inefficient OxPhos.
Either way, there is no argument that elite endurance athletes have an enhanced OxPhos capacity whereas T2Ds have an impaired capacity for fatty acid oxidation, and that T2Ds produce higher levels of lactate at a given workload, echoing the Warburg effect. In fact, when Iñigo San Millán and George Brooks assessed the aerobic capacity in professional endurance athletes in their study, they noticed that the blood lactate levels accumulated in the professional athletes when exercising at approximately 300 watts of output were close to the blood lactate levels observed at rest in people with type 2 diabetes.
Researchers claiming that LDs in the cytoplasm of cancer cells are an indicator of “remarkable abilities to adapt to adverse environmental conditions” — as one group put it — seem to be arguing that LDs in cancer cells are more akin to LDs in the skeletal muscles of endurance athletes. This is in line with the SMT and the belief that cancer cells are more fit than normal cells and that OxPhos are functional in cancer cells. If that’s the case, their claim could be supported by cancer cells with corresponding LDs having nearby mitochondria with enhanced number, structure, function, and OxPhos capacity, similar to what is found in individuals with higher aerobic capacity (discussed here and here).
Instead, Seyfried and colleagues provide a laundry list of citations in their 2020 review showing evidence of the opposite: abnormalities in mitochondrial number, ultrastructure, and function, thus supporting the hypothesis that LD accumulation implies that ATP synthesis via OxPhos is insufficient or impaired and compensatory energy pathways would be necessary for maintaining cancer cell viability. LDs found in the cytoplasm of cancer cells are more akin to what occurs in skeletal muscle cells of individuals with metabolic syndrome and T2D: a pathologic consequence, not a physiological adaption.
In other words, the presence of cytoplasmic LD accumulation and mitochondrial abnormalities in common cancers provides more support for the MMT of cancer and adds to the growing list of inconsitencies of the SMT (see Section 3 of the linked paper).
Upcoming Events with the Author
Previous
Next
LEARN MORE ABOUT JOURNAL CLUB
Bob Kaplan is an independent research analyst. Bob previously served as director of research at Early Medical, contributing to The Drive podcast with Peter Attia and its other properties. Kaplan was a researcher at the Nutrition Science Initiative (NuSI), and an exercise physiologist at the University of Nevada, Las Vegas. His current and previous research interests include meta-research, chronic diseases, bioenergetics, exercise physiology, and nutrition.
Support the Broken Science Initiative.
Subscribe today →
2 Comments
Leave A Comment
You must be logged in to post a comment.
recent posts
What Addiction Medicine Missed – and What Bitten Jonsson’s Work Reveals
The Daley Fix with Matt Shindeldecker & Debbie Wagner



Thank you, Bob!
Thomas Seyfried’s “Cancer as a Metabolic Disease” is a thick read but well worth it. The evidence supporting the MMT of cancer that he provides is difficult to argue with.
His nucleus and mitochondrial transplantation experiments form some of the strongest evidence and are quite easy to understand!
His explanation of gene mutations via ROS of deranged ox phos make a lot more sense to me than the SMT explanation, as well.
Lastly, I find his experiments identifying glutamine as a key fuel source of cancer cells, in addition to glucose, to be quite interesting. Do we know why the cells choose glutamine and not another amino acid?
Thank you to BSI for helping this research along.
Peter –
Thanks for your comment and question.
There are two main reasons why glutamine may be the chosen one.
Quick contextual note. Tom and crew have shown convincing evidence (to me, at least) that glutamine can be a source of ATP independent of oxygen (and therefore OXPHOS) through mitochondrial substrate-level phosphorylation (mSLP) occurring in the TCA cycle.
It goes: [cell] glutamine → [mitochondrial matrix] glutamate → [TCA cycle] alpha-ketoglutarate → succinyl-CoA + ADP → succinate + ATP
1. Glutamine is the most abundant amino acid in humans.
2. Glutamine and glutamate are the only metabolites that don’t expend energy to become succinyl-CoA to make ATP.
Reason #2 is critical. One molecule of glutamine yields one ATP via mSLP. If it uses an ATP to get broken down to succinyl-CoA, the cell just netted zero ATP. This is what happens with other amino acids like isoleucine, valine, thymine, and methionine.
Glutamate also fits the bill as far as #2 goes (and you can convert other AAs like proline and arginine to glutamate), and other AAs break down toward alpha-ketoglutarate (e.g., glycine, serine), but the concentrations of these metabolites are far lower than glutamine, so that’s where reason #1 comes in.
Tom and his colleague Christos cover this in a 2018 paper.
Hope that helps!